Quasi Vivo流动培养促进心肌细胞形成血管结构
——流动培养和明胶支架(3D细胞支架)进行三维培养/3D培养/类器官培养
在再生医学领域,怎样培养出含血管的组织,是未来应用能否成功的关键之一。早期的试验采用生长因子或细胞注射的方法来修补损伤的心脏,但由于注射细胞造成的炎症反应和局部缺血会在体内造成低氧环境,使得注射的细胞定植率低而死亡率高,不能有效地修复损伤的心脏功能。

QV500流动培养系统为接种在明胶支架上的人间充质干细胞(hMSCs)和人心肌祖细胞(hCMPC)提供充足的氧气,促进细胞和营养物质向支架核心内扩散,并能快速有效地排除组织内的代谢废物,促进血管生成,从而形成由血管样和心脏样细胞组成的组织结构密集的适于体内移植的原组织。(Pagliari S, et al. A multistep procedure to prepare pre-vascularized cardiac tissue constructs using adult stem cells, dynamic cell cultures, and porous scaffolds. Frontiers in Physiology. 2014; 5: 210)
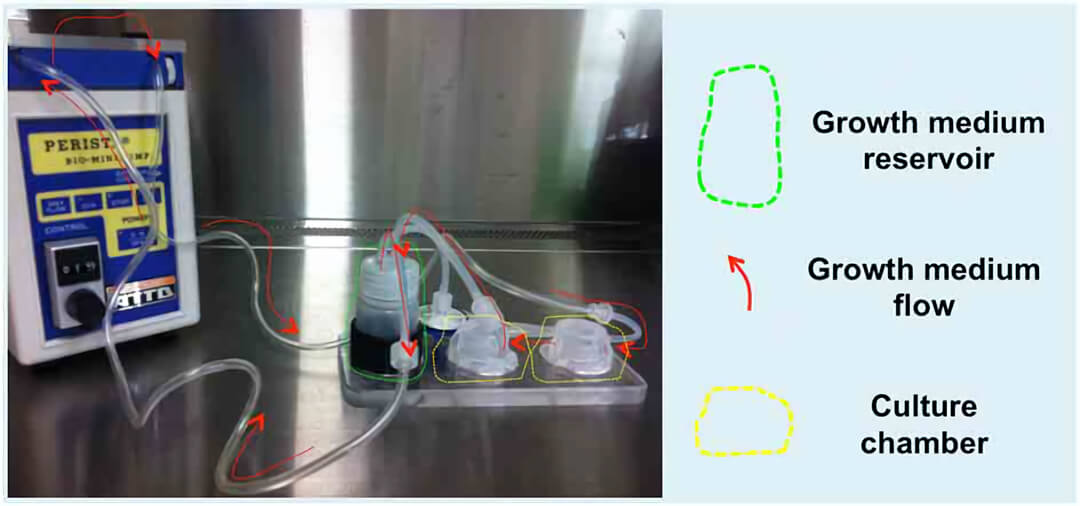
图1. Quasi-Vivo流动培养系统 (QV500型)的蠕动泵将培养基从储液瓶泵到两个串联的培养腔室内,并能保持恒定流速(200μl/min),保证多孔明胶支架内层的培养基流动。
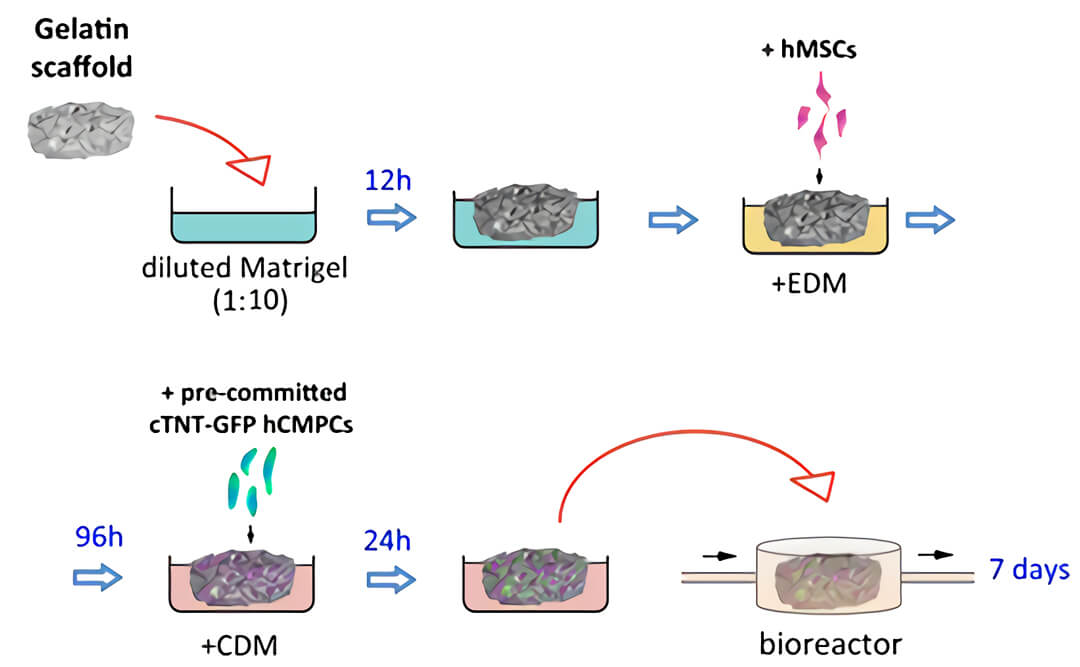
图2. 构建含血管的3D心脏的实验方案示意图。明胶多孔支架(Gelatin scaffold)被浸入稀释的Matrigel中,然后转移至内皮分化培养基(EDM)中。之后将人间充质干细胞(hMSCs)接种在支架上,使人间充质干细胞定殖在支架培养上并向内皮进行分化,96小时后,将在聚苯乙烯细胞培养板用心脏分化培养基(CDM)预先定型2周的心脏TNT-GFP人心肌祖细胞接种于血管化的支架上,用QV500流动培养系统在心脏分化培养基中培养7天。
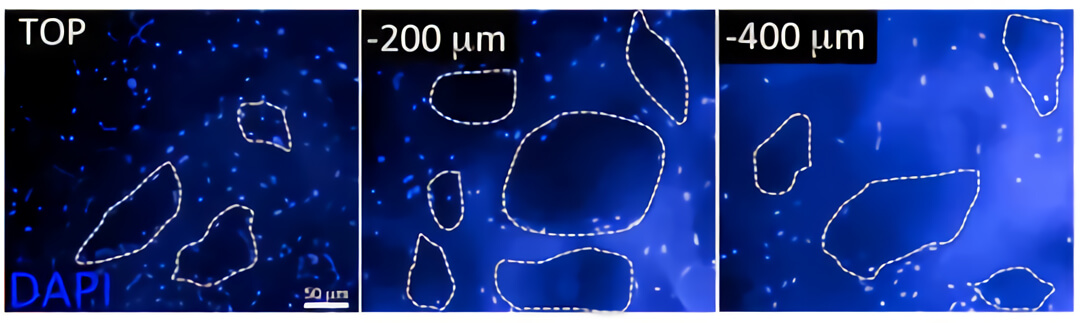
图3. 采用图2的实验方案,对用QV500培养一周后的共培养结构进行检测,发现在支架上有大量细胞定殖。
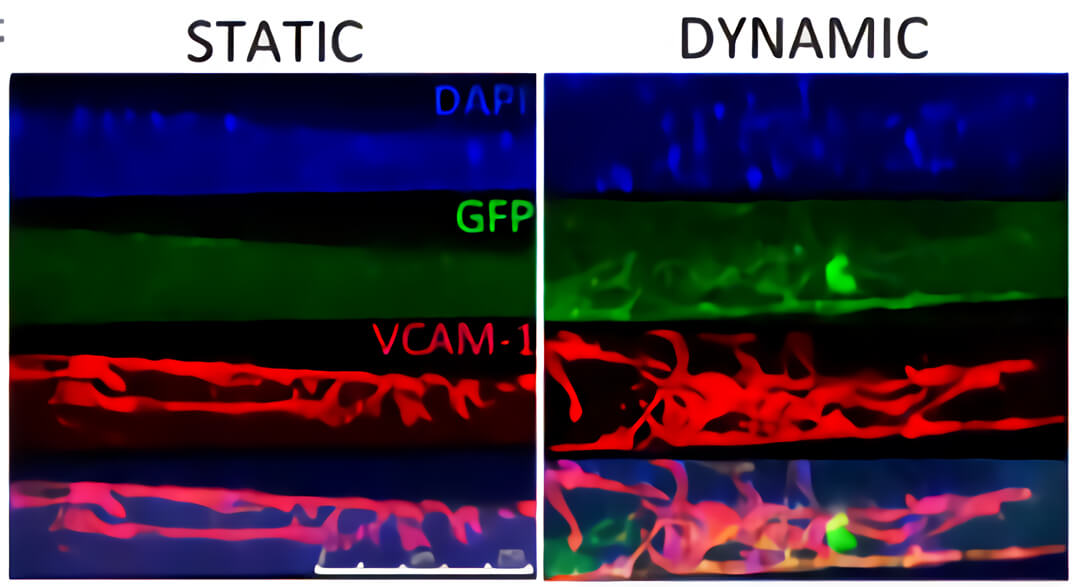
图4. QV500流动培养条件下(DYNAMIC)支架内部浸润了大量的血管样细胞(红色)和人心肌前体细胞(hCMPC)衍生的心肌细胞(绿色),而静态培养条件下(STATIC),细胞大部分分布在支架表面。
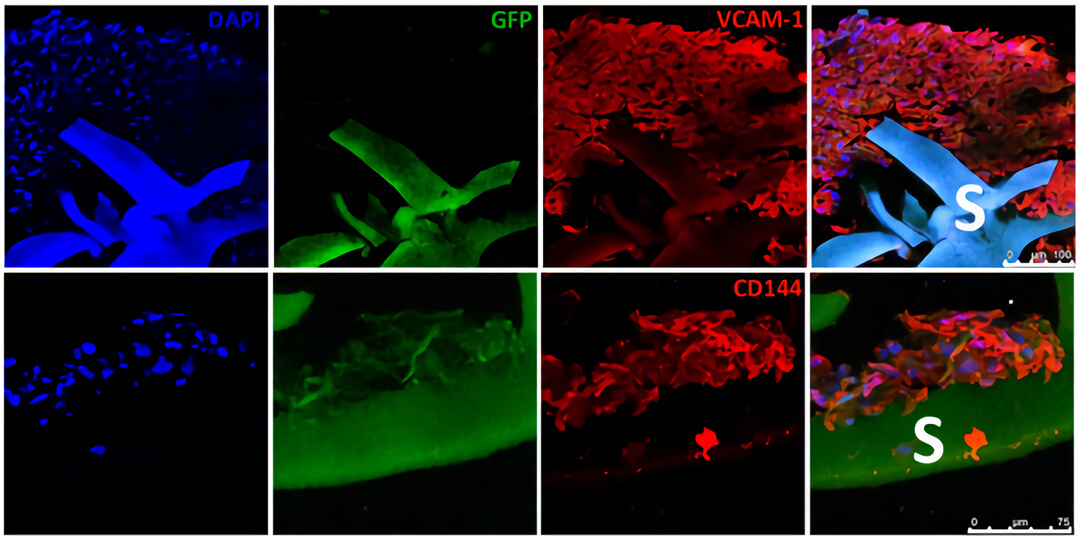
图5. 免疫组化结果显示通过QV500动态培养可以促进心肌样细胞(GFP,绿色)和内皮样细胞(VCAM-1阳性细胞,红色)向支架内部浸润。
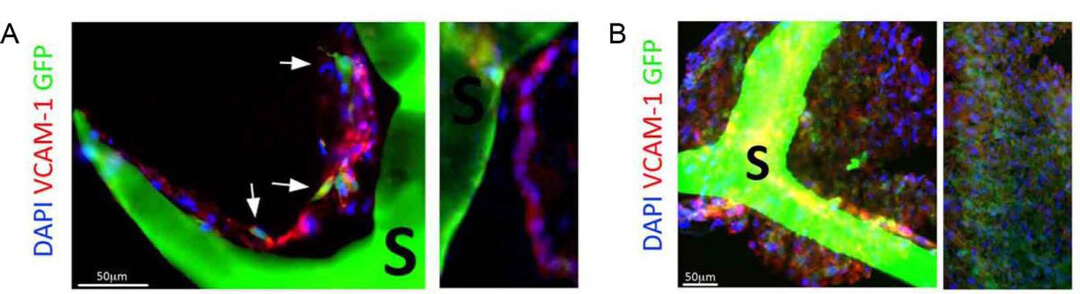
图6. (A) 切片显示QV500流动培养的内皮样细胞(VCAM-1阳性细胞,红色)排列成孔状,形成管状结构,并与心肌样细胞(GFP,绿色)接触。(B)QV500流动培养条件下,支架内广泛的细胞分布导致形成密集组装的多细胞组织,该组织衍生自所用的人间充质干细胞(hMSCs)和人心肌前体细胞(hCMPC)。
总结:在本文中使用的QV500流动培养系统,能增强氧气与营养物质的运输,进而增强工程化心血管组织的活性和功能。
与众不同的Quasi Vivo流动培养系统,让日、美、英、法、瑞士、瑞典等全球70多个研究机构获得了更强大的细胞培养工具,在包括呼吸系统、心血管系统、肝脏、肾脏、肠道、脑组织类器官,以及糖尿病的研究上更进一步。
Quasi Vivo流动培养系统,贴壁细胞培养实验指导
Quasi Vivo参考文献
1.Tommaso S. et al., 2011. Engineering Quasi-Vivo in vitro organ models. Advances in Experimental Medicine and Biology Volume: 745, pp 138-153.
2.Patricia M. et al., 2018. A novel dynamic multicellular co-culture system for studying individual blood-brain barrier cell types in brain diseases and cytotoxicity testing. Scientific Reports Volume: 8, Issue: 1, pp 8784.
3.Basma E. et al. 2020. A dynamic perfusion based blood-brain barrier model for cytotoxicity testing and drug permeation. Scientific Reports Volume: 10, Issue: 1, pp 3788.
4.Miranda A. et al., 2016. A three dimensional (3D) human in vitro blood-brain barrier (BBB). Heart Volume: 102.
5.Buesch S. et al., 2018. A Novel In Vitro Liver Cell Culture Flow System Allowing Long-Term Metabolism and Hepatotoxicity Studies. Applied In Vitro Toxicology Volume: 4, Issue: 3, pp 232-237.
6.Alec O. et al., 2019. Development of an in vitro media perfusion model of Leishmania major macrophage infection. 2019 PLOS ONE Volume: 14, Issue: 7.
7.Sean M. et al., 2017. In-silico Characterisation of the Kirkstall QV900 In-Vitro System for Advanced Cell Culture. 5th International Conference on Computational and Mathematical Biomedical Engineering pp 1174-1177.
8.Ahluwalia A. et al., 2011. Hepatotoxicity of diclofenac in a Quasi-Vivo™ multicompartment bioreactor. oxicology Letters Volume: 205.
9.Tomlinson, L. et al., 2019. In vitro liver zonation of primary rat hepatocytes.Front. Bioeng. Biotechnol., 7(17).
10.Elbakary, B. and Badhan R. K. S, 2020. A dynamic perfusion based blood brain barrier model for cytotoxicity testing and drug permeation. Scientific Reports, 10(1),3788.
11.O’Keefe, A. et al., 2019. Development of an in vitro media perfusion model of Leishmania major macrophage infection. Plos One, 14(7).
12.Miranda-Azpiazu, P. et al., 2018. A novel dynamic multicellular co-culture system for studying individual blood-brain barrier cell types in brain diseases. Scientific Reports, 8, 8784.
13.Chandorkar, P. et al., 2017. Fast-track development of an in vitro 3D lung/immune cell model to study Aspergillus infections. Scientific Reports, 7, 11644.
14.Iori, E. et al., 2012. Glucose and fatty acid metabolism in a 3 tissue in-vitro model challenged with normo- and hyperglycaemia. PLoS ONE, 7(4).
15.Mattei, G., Giusti, S. & Ahluwalia, A., 2014. Design Criteria for Generating Physiologically Relevant In Vitro Models in Bioreactors. Processes, 2(3).
16.Mazzei, D. et al., 2010. A low shear stress modular bioreactor for connected cell culture under high flow rates. Biotechnology and Bioengineering, 106.
17.Nithiananthan, S. et al., 2016. Physiological Fluid Flow Moderates Fibroblast Responses to TGF-β1. Journal of Cellular Biochemistry, 13.
18.Ramachandran, S.D. et al., 2015. In vitro generation of functional liver organoid-like structures using adult human cells. PLoS ONE, 10(10).
19.Rashidi, H. et al., 2016. Fluid shear stress modulation of hepatocyte like cell function. Archives of Toxicology, 90, 7.
20.Vinci, B. et al., 2012. An in vitro model of glucose and lipid metabolism in a multicompartmental bioreactor. Biotechnology Journal, 7.
21.Iori, E. et al., 2012. Glucose and fatty acid metabolism in a 3 tissue in-vitro model challenged with normo- and hyperglycaemia. PLoS ONE, 7(4), pp.1–9.
22.Mattei, G., Giusti, S. & Ahluwalia, A., 2014. Design Criteria for Generating Physiologically Relevant In Vitro Models in Bioreactors. Processes, 2, pp.548–569.
23.Mazzei, D. et al., 2010. A low shear stress modular bioreactor for connected cell culture under high flow rates. Biotechnology and Bioengineering, 106, pp.127–137.
24.Nithiananthan, S. et al., 2016. Physiological Fluid Flow Moderates Fibroblast Responses to TGF-β1. Journal of cellular biochemistry, 13(October), pp.1–13. Available at:
25.Ramachandran, S.D. et al., 2015. In vitro generation of functional liver organoid-like structures using adult human cells. PLoS ONE, 10(10), pp.1–14.
26.Rashidi, H. et al., 2016. Fluid shear stress modulation of hepatocytelike cell function. Archives of Toxicology, pp.3–7.
27.Iori, E. et al., 2012. Glucose and fatty acid metabolism in a 3 tissue in-vitro model challenged with normo- and hyperglycaemia. PLoS ONE, 7(4).
28.Vinci, B. et al., 2011. Modular bioreactor for primary human hepatocyte culture: Medium flow stimulates expression and activity of detoxification genes. Biotechnology Journal, 6, pp.554–564.
29.Tommaso S. et al., 2011. Engineering Quasi-Vivo in vitro organ models. Advances in Experimental Medicine and Biology Volume: 745, pp 138-153.
30.Ahluwalia A. et al., 2011. Hepatotoxicity of diclofenac in a Quasi-Vivo™ multicompartment bioreactor. oxicology Letters Volume: 205.
Quasi Vivo流动培养系统相关文章
-流动培养解决血脑屏障3种贴壁细胞共培养
-流动培养原代肝细胞基因上调
-流动培养提高人支气管上皮细胞分化
-流动培养用于糖尿病模型cross-talk研究
LONZA人原代细胞相关产品
-人骨髓来源间充质干细胞和培养基
-人心室成纤维细胞和培养基
-人心房成纤维细胞和培养基
点击右侧在线咨询,获取Quasi Vivo流动培养系统免费技术支持+报价。
——Quasi Vivo中国独家代理商,北京泽平