Pall iCELLis大规模病毒生产生物反应器
——工艺转换简单,500m2培养面积
美国Pall颇尔公司生产的iCELLis系列固定床生物反应器(iCELLis Fixed-Bed Bioreactors),专为基因治疗、细胞治疗领域设计,是一种全集成、自动化、高细胞密度的一次性生物反应器,用于贴壁细胞、慢病毒LV、腺病毒AV、腺相关病毒AAV大规模生产,包括用于可行性研究和实验室规模生产的iCELLis Nano系统(4m2培养表面积),和工业规模生产的iCELLis 500系统(500m2培养表面积)。

iCELLis生物反应器,使用紧凑型固定床并填充定制微载体,材料采用医药级涤纶超细纤维,可提供高达500m2的培养表面积,相当于3,000只细胞培养转瓶(每只1700cm2)。其培养条件,非常类似传统2D培养,如细胞工厂等,因此可以从传统培养器具直接转换,而无需重新进行大量条件优化,快速推进研究进程,缩短条件摸索的时间。
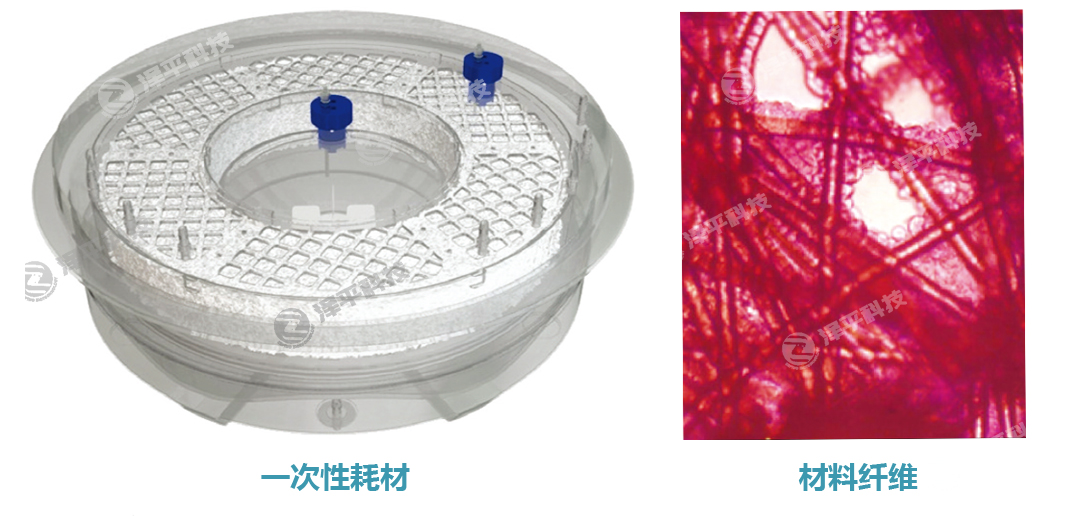
iCELLis可在很低的细胞密度下接种,仅需20个滚瓶即可接种一个iCELLis 500反应器。全集成一次性培养耗材,显著减少人工操作。采用磁力叶轮搅拌,瀑布式供氧,使培养基循环分布均匀,低剪切力,细胞高活力。高度自动化系统,提高批间一致性,令病毒滴度高而一致,高重现性。
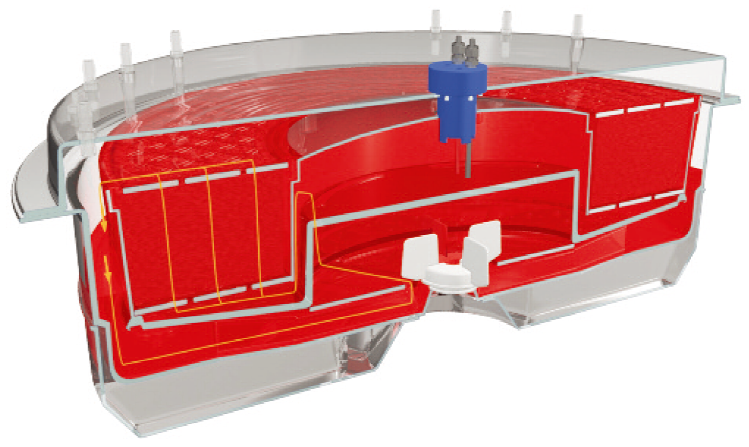
预先安装校准探头,实时监控培养pH、溶氧、二氧化碳含量。配套SCADA操作系统,符合GAMP和FDA 21 CRF Part 11要求,可追溯。
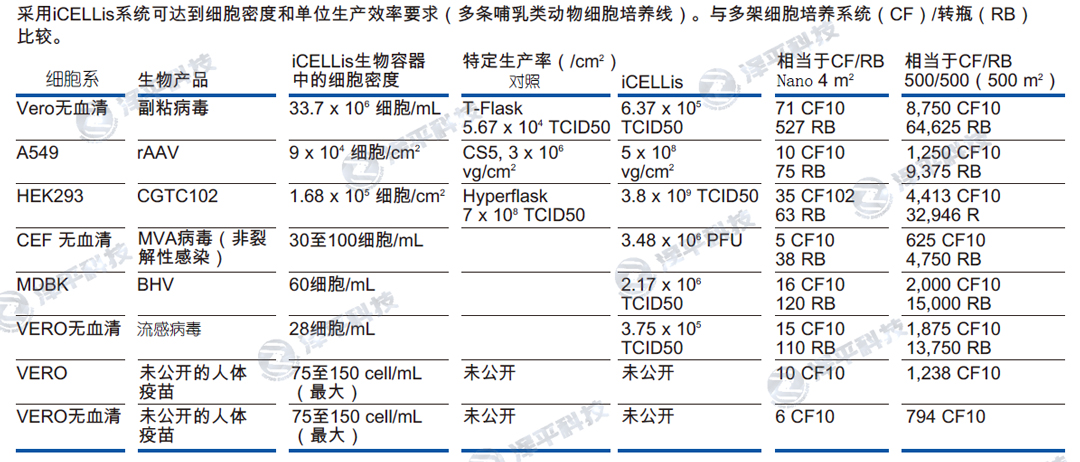
iCELLis生物反应器优势
①与传统2D平板培养条件非常相似,无需额外优化,培养放大性能可预测
②细胞培养过程简化,从培养瓶到最终产品快速生产
③实时监控,可追溯
④全集成,一次性,高重复性,高一致性
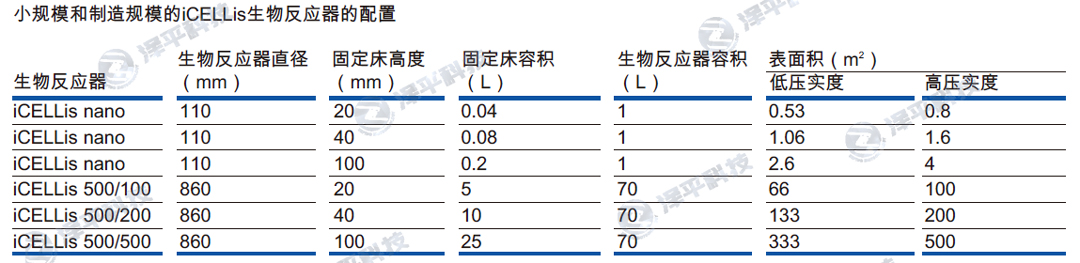
从iCELLis Nano到iCELLis 500线性放大,无需调整工艺。
引用文献
1. Factors affecting kLa in Pall's iCELLis bioreactor. A.Laskowski, S.Gupta, T.Lundeen, et al. Cytotherapy, 2020, Volume 22, Issue 5, Page S150
2. Development and optimization of large scale production of adenoviral vector and autologous insulin producing cell using iCELLis 500 and Xpansion 200 single-use bioreactors. R.Legmann, A.Reniers, N.Kohlstrom, et al. Cytotherapy, 2017, Volume 19, Issue 5, Page S117-S118
3. Intensified Production of Vaccinia-Based Oncolytics in the High Density Cell Respirator (HDCR) Bioreactor Improves Vaccine Logistics and Economics. Cook, Colin, Kang, et al. Molecular Therapy, 2021, page 397-398
4. Platform for the growth and propogation of HEK293 cells and adenovirus viral vector amplification. T.Sanderson, T.Joseph, T.Haq, et al.Cytotherapy, 2019, Volume 21, Issue 5, Pages S24-S25
5. Adherent HEK293T cells cultured in Pall's iCELLis nano bioreactor with OptiPEAK HEK293T blood-free chemically defined media exhibit robust and rapid population doubling times and lentivirus expression. R.Alfano, A.Pennybaker, A.Laskowski, T.Lundeen. Cytotherapy, 2020, Volume 22, Issue 5, Page S151
6. Oncolytic virus scalability affinity chromatography process. A.Thakur, H.Mallory, K.Chung, et al.Cytotherapy, 2020, Volume 22, Issue 5, Page S137
7. Platform approach for the AAV purification process including membrane chromatography for polishing. K.Boenning, J.Huato, A.Hejmowski, et al. Cytotherapy, 2020, Volume 22, Issue 5, Page S186
8. Maintaining excellence in a high volume academic cell therapy production facility through optimization of space utilization, operations, scheduling and staffing. A.Lamontagne, T.A.Colligon, C.Taylor, et al. Cytotherapy, 2020, Volume 22, Issue 5, Pages S149-S150
9. A rapid and reliable qPCR based system to quantitate HEK 293 residual DNA for GMP lot release in gene therapy manufacturing. L.Yan, M.Mateling, K.Norman. Cytotherapy, 2020, Volume 22, Issue 5, Page S186
10. Automated imaging and analysis of colony founding stem and progenitor cells—correlation of early quality attributes with future biological performance.V.R. Mantripragada, V. Luangphakdy, E. Kwee, et al. Cytotherapy, 2017, Volume 19, Issue 5, page S119
更多iCELLis生物反应器信息,欢迎点击右侧在线咨询
——Pall一级代理商,北京泽平